The lower energy product. Inserting the values for Li F into Equation 321b where Q 1 1 Q 2 1 and r 156 pm we find that the energy associated with the formation of a single pair of Li F ions is Ek dfracQ_1Q_2r_0left231 times 1028 Jcancelm right leftdfractext11156.
Describe the energy change associated with ionic bond formation and relate it to stability.

. A short summary of this paper. E k Q 1 Q 2 r 0 231 10 28 J m 1 1 236. A covalent bond is a bond in which two atoms share one or more pairs of electrons.
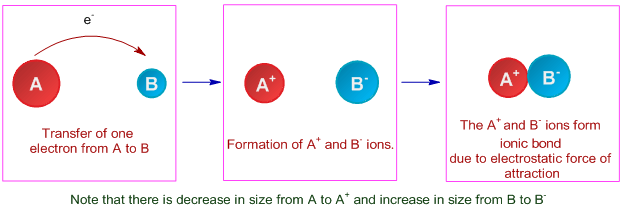
The point at which the potential energy reached its minimum represents the ideal distance between hydrogen atoms for a stable chemical bond to occur. Ionic bond formation is exothermic. Remember that an ionic bond is formed through the transfer of electrons.
When ionic bonds or compound is formed energy is released and it reduces the potential energy of the atom which increases there stablity. Explain how ions form bonds and describe the structure of the resulting compound. I mean let me let me let me repeat that.
The single electrons from each of the two hydrogen atoms. The lower energy product is more stable than the. Describe the energy change associated with ionic bond formation and relate it to stability.
Identify three physical properties of ionic compounds that are associated with ionic bonds and relate them to bond strength. Hence the enthalpy change involved in the process of formation of an ionic compound is negative. Beside this what is the energy change associated with ionic bond formation.
An ionic compound is a compound held together by ionic bonds. 20 Full PDFs related to this paper. Energy is released negative change in enthalpy when forming a bond.
Given that the observed gas-phase internuclear distance is 236 pm the energy change associated with the formation of an ion pair from an Na g ion and a Cl g ion is as follows. So Ill explain it to you right now to several factors for the formation of ionic bond. Oh no compound formed.
Examples of ionic compounds include pyrite FeS 2. The point at which the potential energy reached its minimum represents the ideal distance between hydrogen atoms for a stable chemical bond to occur. The formation of ionic compounds from positive and negative ions is exothermic.
The lower-energy product is more stable than the original reactants. The formation of ionic compounds from positive and negative ions is exothermic. Describe the energy change associated with ionic bond formation and relate it to.
To quantitatively describe the energetic factors involved in the formation of an ionic bond. This type of chemical bond is called a covalent bond. The formation of an ionic compound is an exothermic process.
Questions 12Explain how an ionic compound made up of charged particles can be neutral. Whi And which has the greater electron affinity carbon or nitrogen. So an ionic compound is much more stable when there is a lot more energy because there is a higher bond formation energy and its therefore harder to break them apart compared to compared to those with low energies those with a lot less energy.
Ionic bond formation is exothermic. Describe the energy change associated with ionic bond formation and relate it to stability. The enthalpy change involved in the formation of one mole of an ionic crystal from its constituent gaseous positive and negative ion is called lattice energy.
Ionic Compounds and Metals BIG Idea Atoms in ionic compounds are held together by - chemical bonds formed by the Ca2 CO32 attraction of oppositely charged ions. So um this bond formacion energy the energy they have to form a bond is ah lot higher. Okay what are matters matters are givers of electrons.
This type of chemical bond is called a covalent bond. Identify three physical properties of ionic compounds that are associated with ionic bonds and relate them to bond strength. Up to 24 cash back 13.
What is an ionic bond. So if they have low ionization in therapy which means they can be ionized easily and they will release the electrons very easily. A covalent bond is a bond in which two atoms share one or more pairs of electrons.
The total positive charge of the cations in the compound equals the total negative charge of the anions in the compound. Pm times 1012 cancelmpm right148 times. Breaking a bond requires the input of energy positive change in enthalpy.
Bond energies of the given bonds are as follows- Si-F 582 kJmol Si-C 360 kJmol Si-O 452 kJ. Explain how ions are formed how they form bonds and describe the structure of the resulting compound. Bond enthalpy or dissociation energy is defined as the standard enthalpy change when a bond is cleaved by homolysis with reactants and products of the homolysis reaction at 0 K absolute zero.
The third will be hi lattice and Calgary. Describe the energy change associated with ionic bond formation and relate it to stability. Describe the energy change associated with ionic bond formation and relate it to stability.
13Describe the energy change associated with ionic bond formation and relate it to stabillity. Lattice energy or Lattice Enthalpy. Calcium carbonate CaCO 3 71 Ion Formation MAIN Idea Ions are formed when atoms gain or lose valence.
The single electrons from each of the two hydrogen atoms. The attraction from positive and negative ions form a more stable system with a lower engergy than the individual ions. Describe the energy change associated with ionic bond formation and relate it to stability.
Identify three physical properties of ionic compounds that are associated with ionic bonds Ionic compounds exist as crystals have high. Section 21 Chemical Compounds explained that ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Ionic bond formation is exothermic.
14Identify three physical properties of ionic compounds that are associated with ionic bonds and related them to bond strength.
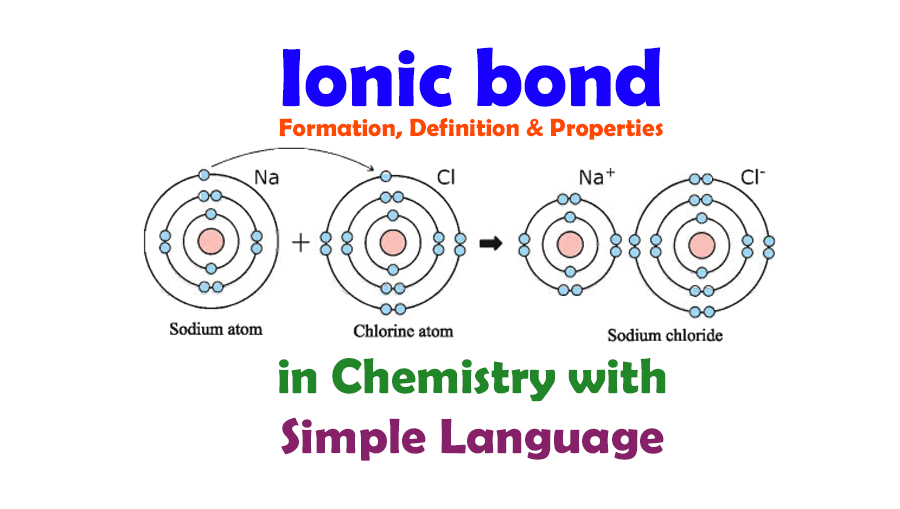
Ionic Bond And Ionic Bond Formation Definition Properties In Chemistry Tuition Tube

0 Comments